Atomic Structure
According to the modern theory, matter is electrical in nature. All the materials are composed of very small particles called atoms. The atoms are the building blocks of all matter. An atom consists of a central nucleus of positive charge around which small negatively charged particles, called electrons, revolve in different paths or orbits.
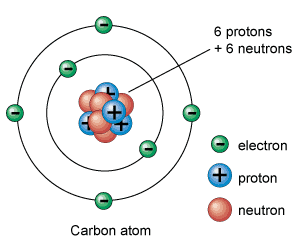
Nucleus.
It is the central part of an atom and contains protons and neutrons. A proton is a positively charged particle, while a neutron has the same mass as a proton but is neutral in charge. Therefore, the nucleus of an atom is positively charged. The sum of protons and neutrons constitutes the entire weight of an atom and is called atomic weight. It is because the particles in the extra nucleus (i.e., electrons) have negligible weight as compared to protons or neutrons.
atomic weight = no. of protons + no. of neutrons
Extra nucleus.
It is the outer part of an atom and contains electrons only. An electron is a negatively charged particle having negligible mass. The charge on an electron is equal but opposite to that on a proton. Also, the number of electrons is equal to the number of protons in an atom under ordinary conditions. Therefore, an atom is neutral as a whole. The number of electrons or protons in an atom is called the atomic number, i.e.
atomic number = no. of protons or electrons in an atom
The electrons in an atom revolve around the nucleus in different orbits or paths. The number and arrangement of electrons in any orbit are determined by the following rules :
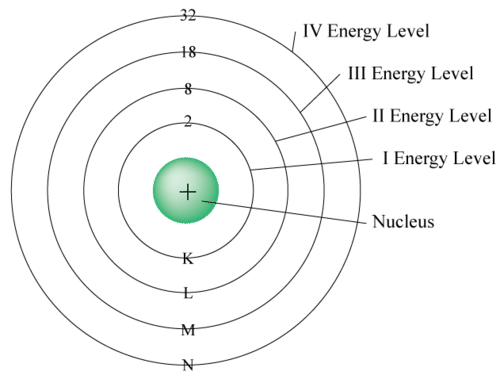
- The number of electrons in any orbit is given by \(2n^{2}\) where n is the number of the orbit. For example,
- the First orbit contains \(2 \times 12 = 2\) electrons,
- the Second orbit contains \(2 \times 22 = 8\) electrons,
- the Third orbit contains \(2 \times 32 = 18\) electrons, and so on.
- The last orbit cannot have more than 8 electrons.
- The last but one orbit cannot have more than 18 electrons.
Structure of Elements
All atoms are made up of protons, neutrons, and electrons. The difference between various types of elements is due to the different numbers and arrangements of these particles within their atoms. For example, the structure of a copper atom differs from that of a carbon atom, and hence the two elements exhibit distinct properties.
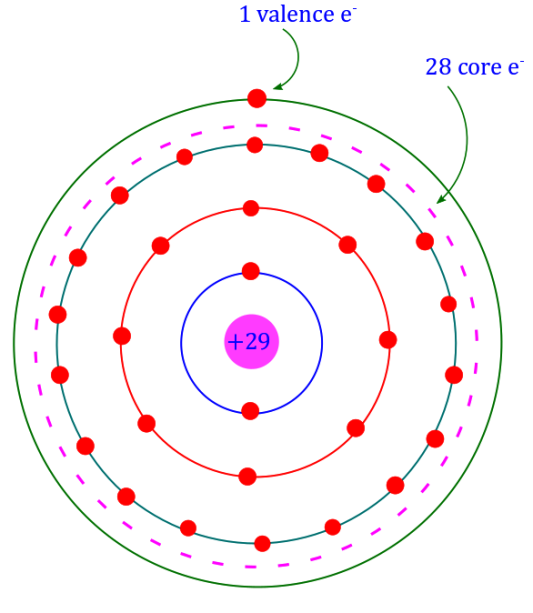
The atomic structure can be easily built up if we know the atomic weight and atomic number of the element. Thus, taking the case of a copper atom,
- Atomic weight = 64
- Atomic number = 29
and
- No. of protons = No. of electrons = 29
- No. of neutrons = 64 − 29 = 35
The figure shows the structure of a copper atom. It has 29 electrons, which are arranged in different orbits as follows. The first orbit will have 2 electrons, the second 8 electrons, the third 18 electrons, and the fourth orbit will have 1 electron. The atomic structure of all known elements can be shown in this way.
The Electron
Since electronics deals with tiny particles called electrons, these small particles require detailed study. An electron is a negatively charged particle having negligible mass. Some of the important properties of an electron are :
- Charge on an electron, e = \(1.602 × 10^{−19}\) coulomb
- Mass of an electron, m = \(9.0 × 10^{−31}\) kg
- Radius of an electron, r = \(1.9 × 10^{−15}\) metre
The ratio e/m of an electron is \(1.77 \times 10^{11}\) coulombs/kg. This means that the mass of an electron is very small compared to its charge. It is due to this property of an electron that it is very mobile and is greatly influenced by electric or magnetic fields.
Energy of an Electron
An electron moving around the nucleus possesses two types of energies, viz. kinetic energy due to its motion and potential energy due to the charge on the nucleus. The total energy of the electron is the sum of these two energies. The energy of an electron increases as its distance from the nucleus increases. Thus, an electron in the second orbit possesses more energy than an electron in the first orbit; an electron in the third orbit has higher energy than an electron in the second orbit. It is clear that electrons in the last orbit possess very high energy as compared to the electrons in the inner orbits. These last orbit electrons play an important role in determining the physical, chemical, and electrical properties of a material.