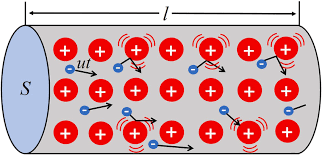
Free Electrons
The valence electrons of different materials possess different energies. The greater the energy of a valence electron, the lesser it is bound to the nucleus. In certain substances, particularly metals, the valence electrons possess so much energy that they are very loosely attached to the nucleus. These loosely attached valence electrons move at random within the material and are called free electrons.
The valence electrons which are very loosely attached to the nucleus are known as free electrons.
The free electrons can be easily removed or detached by applying a small amount of external energy. As a matter of fact, these are the free electrons which deter mine the electrical conductivity of a material. On this basis, conductors, insulators and semiconductors can be defined as under :
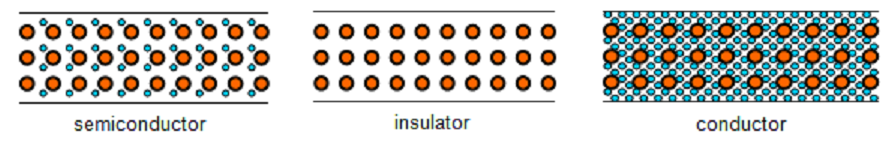
- (i) A conductor is a substance which has a large number of free electrons. When potential differ ence is applied across a conductor, the free electrons move towards the positive terminal of supply, constituting electric current.
- (ii) An insulator is a substance which has practically no free electrons at ordinary temperature. Therefore, an insulator does not conduct current under the influence of potential difference.
- (iii) A semiconductor is a substance which has very few free electrons at room temperature. Consequently, under the influence of potential difference, a semiconductor practically conducts no current.