Research
Research Activities in the Group
The following Domains of research are focused in the Artificial Photosynthesis Group.
-
CO2 reduction and CO2 Hydrogenation
-
Dehydrogenation of HCOOH
-
NH3 Oxidation
-
Proton reduction
-
Water Oxidation
-
Electrochemical Transformations of Organic Compounds
RESERCH HIGHLIGHTS (NEWS & PRESS RELEASE)
-
News on CO2 Hydrogenation (Prabhat Khabar, Hindustan)
-
NEWS: Hindustan; Dainik Bhskar; Prabhat Khabar 01; Prabhat Khabar 02 on H2 from NH3.
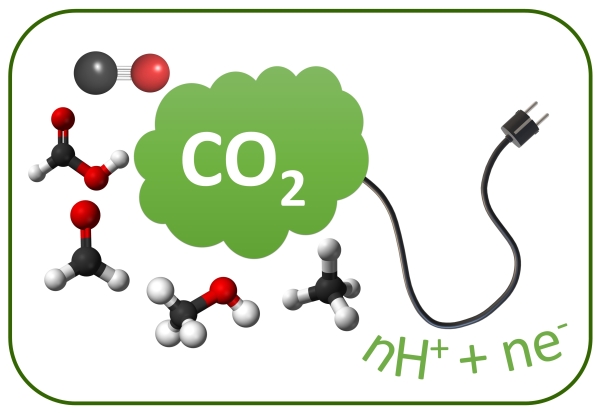
CO2 Reduction and Hydrogenation.
Homogeneous catalysis involves the use of a catalyst that is in the same phase (typically liquid) as the reactants to facilitate a chemical reaction. In the context of CO2 reduction, homogeneous catalysts can play a significant role in converting carbon dioxide into useful chemicals and fuels, thereby addressing environmental concerns related to excess atmospheric CO2.Homogeneous catalysis involves the use of a catalyst that is in the same phase (typically liquid) as the reactants to facilitate a chemical reaction. In the context of CO2 reduction, homogeneous catalysts can play a significant role in converting carbon dioxide into useful chemicals and fuels, thereby addressing environmental concerns related to excess atmospheric CO2.
- Route: Electro, Phtochemical and Thermal
- Catalyst: Transition metal catalysts
- Method: Homogeneous
- Focus: C1 and C2 Products
- Phase: Solution and Gas pase reactions
- Mechanism: DFT and Intermediate trap
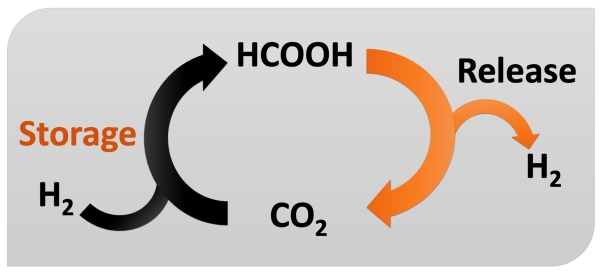
HCOOH dehydrogenation.
Homogeneous catalytic formic acid (HCOOH) dehydrogenation converts formic acid into hydrogen gas (H2) and carbon dioxide (CO2) with a soluble catalyst. This reaction is of particular interest because of its potential applications in hydrogen storage and generation. The catalysts utilized are typically transition metal complexes based on ruthenium, iridium, or iron, which function under mild conditions while providing good selectivity and efficiency. During the dehydrogenation process, the metal catalyst helps to break the C-H and O-H bonds in formic acid, resulting in the release of hydrogen gas. This process is environmentally friendly, producing just CO2 and H2, and shows promise for sustainable energy systems.
- Route: Thermal
- Catalyst: Transition metal catalysts
- Method: Homogeneous
- Focus: CO2 and H2
- Phase: Solution and Gas pase reactions
- Mechanism: DFT and Intermediate trap
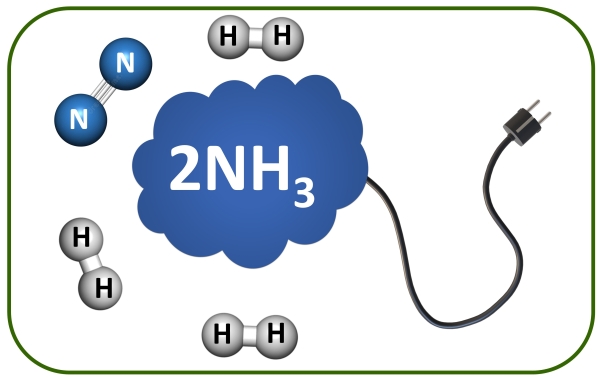
NH3 Oxidation.
The oxidation of ammonia (NH3) to hydrogen (H2) using homogeneous catalysis is an innovative approach in the field of sustainable energy and green chemistry. This process leverages molecular catalysts dissolved in solution to facilitate the oxidation of NH3, a compound rich in hydrogen, to produce H2, a clean and efficient fuel. Basic Principles The catalytic oxidation of NH3 typically involves the dehydrogenation of NH3 to nitrogen gas (N2) and hydrogen gas (H2). The overall reaction is: 2NH3 → N2 + 3H2
- Route: Electrochemical
- Catalyst: Transition metal catalysts
- Method: Homogeneous
- Focus: N2 and H2
- Phase: Solution and Gas pase reactions
- Mechanism: DFT and Intermediate trap
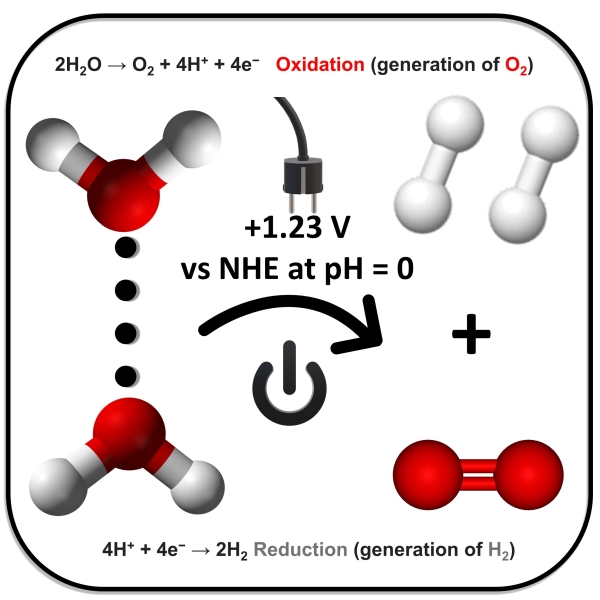
Water Oxidation and Proton Reduction.
In artificial photosynthesis and renewable energy research, homogeneous catalytic water oxidation is vital. This process involves oxidizing water molecules (H2O) to produce oxygen gas (O2), protons (H⁺), and electrons. This reaction is necessary for producing the oxygen needed in water-splitting processes, which eventually manufacture hydrogen fuel from water using solar energy. Catalysts for homogeneous water oxidation are typically transition metal complexes based on ruthenium, iridium, or manganese that act in a homogeneous solution. These catalysts help in the four-electron oxidation of water by providing an effective channel for electron and proton transfer. The creation of effective and stable homogeneous catalysts for water oxidation presents a significant challenge due to the reaction's demanding parameters, which include large overpotentials and stability in oxidative environments. Successful advances in this field show promise for long-term energy solutions, such as efficient solar energy conversion and storage.
- Route: Electro and Photochemical
- Catalyst: Transition metal catalysts
- Method: Homogeneous
- Focus: O2 and H2
- Phase: Solution pase reactions
- Mechanism: DFT and Intermediate trap
